Pharma 4.0 Market Introduction
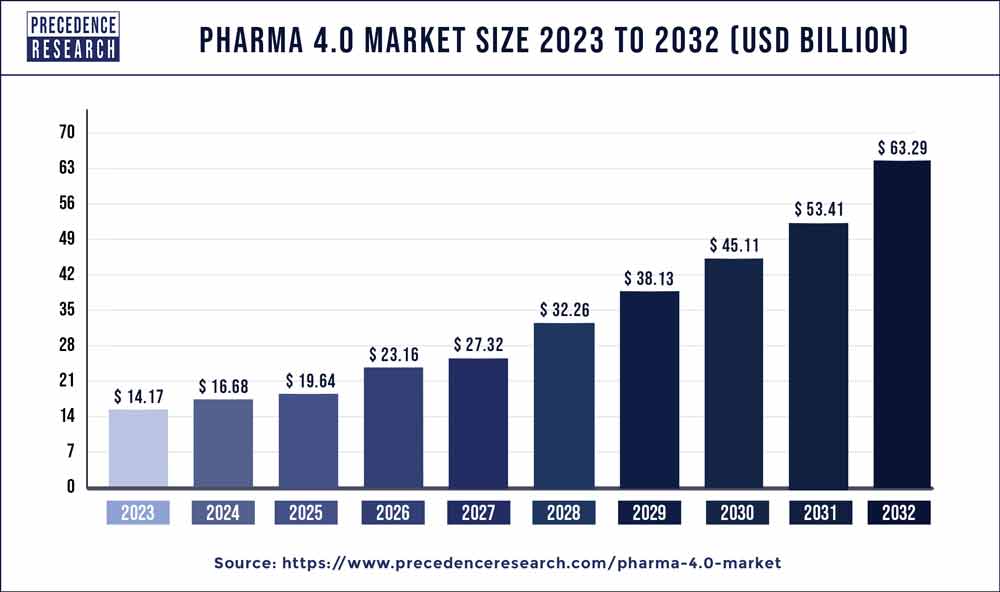
The global pharma 4.0 market size is anticipated to reach around USD 63.29 billion by 2032 from USD 14.17 billion in 2023 and is poised to grow at a CAGR of 18.1% during the forecast period from 2024 to 2032.
Pharma 4.0 marks a paradigm shift in how pharmaceutical companies operate and deliver value across the healthcare ecosystem. Unlike its predecessors—Pharma 1.0 characterized by manual labor, Pharma 2.0 with automation in manufacturing, and Pharma 3.0 integrating digital technologies—Pharma 4.0 leverages advanced digital tools such as artificial intelligence (AI), big data analytics, Internet of Medical Things (IoMT), and blockchain to drive innovation and efficiency.
Pharma 4.0 Market by Technology
- IoT Dominance: IoT holds a substantial share in the Pharma 4.0 market, enabling continuous monitoring and feedback loops for process improvement.
- Real-time Data: Real-time data capture and analysis help in identifying inefficiencies and implementing corrective actions swiftly, enhancing product quality and reducing waste.
- Agility and Innovation: Pharma 4.0 emphasizes agility, adaptability, and innovation, crucial for responding to market dynamics and customer needs effectively.
Get a Sample: https://www.precedenceresearch.com/sample/3187
Pharma 4.0 Market by Application
- Manufacturing Dominance: The manufacturing segment leads, leveraging Pharma 4.0 for real-time monitoring, quality assurance, and regulatory compliance.
- Integrated Data: Integration of data from various sources facilitates advanced analytics and AI-driven insights, optimizing processes and predicting issues preemptively.
Pharma 4.0 Market by End-User
- Pharmaceutical Companies: These companies dominate the market, driven by increased adoption of advanced technologies to boost operational efficiency and product quality.
- Productivity and Quality: Pharma 4.0 aims to enhance productivity and quality management, crucial amid rising global demand for pharmaceuticals.
Read Report: Citric Acid Market Size to Worth USD 5.12 Bn by 2032
Pharma 4.0 Market Driving Technologies
- Artificial Intelligence and Machine Learning
AI and ML are revolutionizing drug discovery and development processes. Pharmaceutical companies are using AI-powered algorithms to sift through vast amounts of data, accelerating the identification of potential drug candidates and predicting their efficacy. For example, Insilico Medicine used AI to identify new molecules for potential treatments, significantly reducing the time and cost traditionally associated with drug discovery.
Moreover, machine learning models are employed in predictive analytics to forecast patient responses to treatments, enabling personalized medicine approaches. This capability not only enhances patient outcomes but also optimizes clinical trial designs by identifying suitable patient cohorts more efficiently.
- Big Data and Analytics
The pharmaceutical industry generates enormous volumes of data—from clinical trials and electronic health records to supply chain operations and patient feedback. Big data analytics harnesses this wealth of information to extract actionable insights, improving decision-making processes at every stage of drug development and commercialization.
For instance, companies like Pfizer utilize big data analytics to analyze real-world evidence (RWE) and validate drug effectiveness in diverse patient populations beyond clinical trial settings. This approach not only supports regulatory submissions but also informs post-market surveillance strategies, ensuring continuous product improvement and patient safety.
- Internet of Medical Things (IoMT)
The IoMT encompasses interconnected medical devices, wearables, and remote monitoring systems that collect real-time health data. In Pharma 4.0, IoMT devices enable continuous patient monitoring, facilitating early disease detection and intervention. These devices also enhance medication adherence through smart pill dispensers and personalized treatment reminders, thereby improving patient compliance and health outcomes.
Furthermore, IoMT technologies streamline clinical trials by remotely capturing patient data and minimizing site visits, reducing trial costs and timelines. This approach enhances patient recruitment and retention rates while maintaining data integrity and regulatory compliance.
- Blockchain Technology
Blockchain’s decentralized ledger technology ensures transparency, traceability, and security across pharmaceutical supply chains. By recording every transaction—from drug manufacturing and distribution to patient prescriptions and outcomes—blockchain minimizes counterfeit drugs, enhances supply chain efficiency, and strengthens regulatory compliance.
For instance, IBM’s Blockchain Platform collaborates with pharmaceutical giants like Merck to create an immutable record of drug provenance and authenticity, combating the global issue of counterfeit medications. Such transparency instills trust among stakeholders, improves patient safety, and supports pharmacovigilance efforts worldwide.
Digital Transformation in Drug Development
Pharma 4.0 accelerates drug development through innovative approaches that streamline processes and optimize resource allocation. Virtual drug trials, for example, leverage digital platforms to conduct clinical studies remotely, eliminating geographical barriers and reducing patient recruitment timelines.
Similarly, computational modeling and simulation techniques simulate drug interactions within biological systems, predicting efficacy and toxicity profiles early in the development cycle. This computational approach expedites candidate selection, mitigates risks, and optimizes therapeutic outcomes before proceeding to costly and time-consuming clinical trials.
Personalized medicine represents another hallmark of Pharma 4.0, tailoring treatments to individual genetic profiles and biomarkers. Genetic testing and biomarker identification enable clinicians to prescribe targeted therapies, maximizing efficacy while minimizing adverse effects. Companies like Roche’s Foundation Medicine pioneer personalized oncology treatments, analyzing tumor genomes to recommend precise therapies tailored to each patient’s molecular profile.
Manufacturing and Supply Chain Innovations
In pharmaceutical manufacturing, smart technologies drive efficiency and flexibility across production lines. IoT-enabled sensors monitor critical process parameters in real-time, ensuring consistent product quality and compliance with Good Manufacturing Practices (GMP). Automated robotic systems handle intricate tasks such as drug packaging and labeling, reducing human error and operational costs.
Supply chain optimization remains pivotal in Pharma 4.0, as companies deploy predictive analytics to forecast demand, optimize inventory levels, and mitigate supply disruptions. These analytics-driven insights enhance supply chain resilience, expedite time-to-market, and ensure uninterrupted drug availability for patients worldwide.
Moreover, digital twins—virtual replicas of physical assets—simulate manufacturing processes and supply chain operations, allowing pharmaceutical companies to identify optimization opportunities and refine operational strategies proactively. This digital modeling approach optimizes resource utilization, minimizes waste, and supports sustainable manufacturing practices in line with global regulatory standards.
Healthcare Ecosystem and Patient-Centric Approaches
Pharma 4.0 fosters collaboration across the healthcare ecosystem, promoting patient-centric care through telemedicine and digital health platforms. Telemedicine services expanded exponentially during the COVID-19 pandemic, facilitating remote consultations, diagnoses, and treatment plans while ensuring patient safety and continuity of care.
Digital health platforms integrate patient data from various sources—IoMT devices, electronic health records, and wearable sensors—to provide holistic insights into individual health profiles. This comprehensive data aggregation empowers healthcare providers to deliver personalized interventions, monitor treatment adherence, and optimize therapeutic outcomes in real-time.
However, ensuring patient data privacy and cybersecurity remains paramount in Pharma 4.0. Regulatory frameworks like the General Data Protection Regulation (GDPR) and Health Insurance Portability and Accountability Act (HIPAA) mandate stringent data protection measures, safeguarding patient confidentiality and maintaining trust in digital healthcare services.
Pharma 4.0 Market Trends and Future Outlook
The global adoption of Pharma 4.0 is accelerating across regions, driven by increasing investments in digital infrastructure, regulatory support for technological innovation, and rising demand for personalized healthcare solutions. North America leads the Pharma 4.0 market, fueled by robust R&D investments, strategic collaborations between pharmaceutical companies and technology firms, and supportive regulatory frameworks promoting innovation.
Europe follows closely, embracing digital health initiatives and fostering cross-border collaborations to enhance healthcare interoperability and patient outcomes. Meanwhile, Asia-Pacific emerges as a key growth region, propelled by expanding healthcare access, rising chronic disease burden, and government initiatives promoting digital health transformation.
Despite its transformative potential, Pharma 4.0 faces challenges such as regulatory compliance complexities, data interoperability issues, and workforce upskilling requirements. Overcoming these barriers necessitates collaborative efforts between industry stakeholders, policymakers, and regulatory authorities to establish harmonized standards and facilitate technology adoption seamlessly.
Looking ahead, emerging technologies like 5G connectivity and edge computing promise to further revolutionize Pharma 4.0, enabling real-time data transmission, enhanced telemedicine capabilities, and advanced AI-driven diagnostics. These innovations will redefine patient care delivery, empower healthcare providers with actionable insights, and pave the way for future advancements in pharmaceutical research, manufacturing, and patient engagement.
Pharma 4.0 Market Companies
- Microsoft Corporation
- Oracle Corporation
- ABB
- Honeywell International Inc.
- Cisco Systems, Inc.
- Siemens Healthcare GmbH
- GE Healthcare
- IBM Corporation
- Amazon Web Services, Inc.
Segments Covered in the Report:
By Technology
- Cloud Computing
- Artificial Intelligence (AI)
- Big Data Analytics
- Internet of Things (IoT)
By Application
- Drug Discovery and Development
- Clinical Trials
- Manufacturing
By End-User
- Pharmaceutical Companies
- Biotechnology Companies
- CROs and CMOs
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
