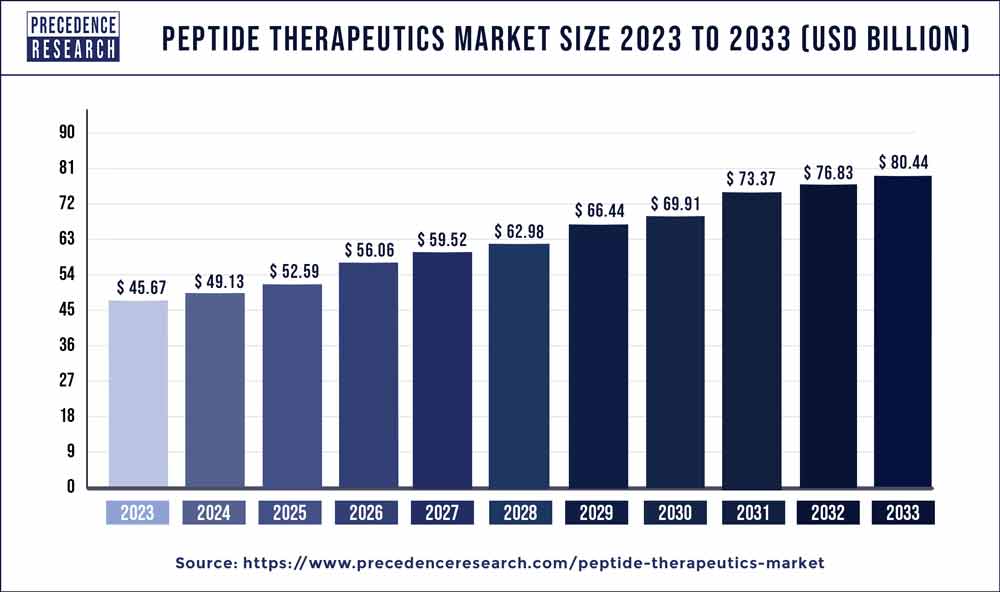
The global peptide therapeutics market size is anticipated to reach around USD 80.44 billion by 2033 from USD 45.67 billion in 2023 and is poised to grow at a CAGR of 5.63% during the forecast period from 2024 to 2033.
Peptide Therapeutics Market Regional Analysis
North America:
-
- United States: Dominates the North American market due to a strong biotechnology sector and significant investment in research and development.
- Canada: Showing steady growth with increasing government initiatives and funding for biopharmaceutical research.
Europe:
-
- Western Europe: Leading in peptide therapeutics adoption, supported by robust healthcare infrastructure and high R&D investments.
- Eastern Europe: Emerging market with growing pharmaceutical sector and improving regulatory environment.
Asia Pacific:
-
- China: Rapidly expanding market driven by large patient population and government initiatives to improve healthcare access.
- India: Significant growth potential due to cost-effective manufacturing capabilities and increasing focus on biotechnology.
- Japan: Mature market with a strong emphasis on innovative therapies and advanced healthcare technologies.
Latin America:
-
- Brazil: Largest market in the region, driven by economic development and increasing healthcare expenditures.
- Mexico: Growing pharmaceutical industry with rising demand for advanced therapeutics.
Middle East & Africa:
-
- GCC Countries: High healthcare spending and investment in infrastructure supporting market growth.
- South Africa: Emerging as a key market with expanding healthcare access and increasing prevalence of chronic diseases.
Get a Sample: https://www.precedenceresearch.com/sample/2548
Peptide Therapeutics Market Dynamics
Drivers
- Growing Prevalence of Chronic Diseases:
- The increasing incidence of chronic diseases such as cancer, diabetes, and cardiovascular diseases is a significant driver for the peptide therapeutics market. These conditions require advanced and targeted treatments, and peptides offer promising solutions due to their specificity and potential for tailored therapies.
- Advancements in Peptide Drug Delivery Technologies:
- Technological advancements in peptide synthesis, formulation, and drug delivery systems have revolutionized the field. Novel techniques such as lipidation, conjugation to carrier molecules, and development of peptide-drug conjugates have enhanced the stability, bioavailability, and targeted delivery of peptide-based drugs. These advancements are crucial for improving efficacy and patient outcomes.
- Rising Investment in R&D:
- Pharmaceutical companies and research institutions are increasingly investing in R&D focused on peptide therapeutics. This investment is driven by the need to explore new peptide sequences, optimize delivery methods, and conduct clinical trials to demonstrate safety and efficacy. The result is a growing pipeline of innovative peptide drugs addressing diverse therapeutic areas.
- Favorable Regulatory Environment:
- Regulatory bodies such as the FDA and EMA have implemented expedited approval pathways and incentives like orphan drug designations for peptide therapeutics. These initiatives streamline the regulatory process, reducing time-to-market for new peptide drugs and encouraging innovation in the industry.
- Increasing Biopharmaceutical Industry:
- The biopharmaceutical sector’s expansion has provided a robust infrastructure for peptide therapeutics development and commercialization. Biotech companies specializing in peptide research and production benefit from advanced manufacturing capabilities and expertise, facilitating the translation of research into therapeutic products.
Restraints
- High Production Costs:
- The synthesis, purification, and formulation of peptides can be costly, impacting overall drug development expenses and the final pricing of peptide therapeutics. High production costs pose a barrier to widespread adoption and affordability, particularly for healthcare systems with budget constraints.
- Short Half-life of Peptides:
- Peptides often exhibit a short half-life in the bloodstream due to enzymatic degradation and rapid clearance. This necessitates frequent dosing or the development of innovative delivery systems (such as sustained-release formulations or peptide modifications) to maintain therapeutic levels in the body, which can be technically challenging and costly.
- Complex Regulatory Landscape:
- Regulatory approval for peptide therapeutics involves rigorous safety and efficacy assessments, including extensive preclinical and clinical studies. The complex regulatory landscape, with stringent requirements for clinical trial design and data submission, can prolong development timelines and increase costs for companies seeking market approval.
- Competition from Small Molecule Drugs and Biologics:
- Peptide therapeutics face competition from well-established small molecule drugs and biologics in various therapeutic areas. Small molecules often offer simpler manufacturing processes and lower costs, while biologics may have longer half-lives and established market presence. This competitive landscape challenges the market penetration and growth of peptide-based treatments.
- Challenges in Drug Delivery:
- Effective delivery of peptides to target tissues or cells remains a significant challenge. Peptides are susceptible to enzymatic degradation and may face barriers crossing cellular membranes or the blood-brain barrier. Innovative drug delivery systems, such as nanoparticles or peptide conjugates, are being developed to enhance peptide stability, improve bioavailability, and optimize therapeutic outcomes.
Opportunities
- Development of Novel Peptides:
- There is considerable potential for discovering and developing novel peptides with unique pharmacological profiles and therapeutic targets. Advances in peptide library screening, computational design, and structural modification techniques enable the creation of peptides that specifically interact with disease targets, addressing unmet medical needs.
- Personalized Medicine:
- The shift towards personalized medicine presents an opportunity for peptide therapeutics. Peptides can be tailored to target specific molecular pathways or patient populations, offering individualized treatment options with potentially enhanced efficacy and reduced side effects compared to traditional therapies.
- Emerging Markets:
- Expansion into emerging markets with growing healthcare infrastructure and increasing healthcare expenditures offers significant growth opportunities for peptide therapeutics. These regions present untapped markets for novel therapies, driven by rising disease prevalence and expanding access to innovative treatments.
- Collaborations and Partnerships:
- Strategic collaborations between pharmaceutical companies, biotech firms, academic institutions, and contract research organizations (CROs) can accelerate innovation in peptide therapeutics. Partnerships facilitate shared expertise, resources, and access to novel technologies, expediting drug discovery, development, and commercialization efforts.
- Innovations in Drug Delivery Systems:
- Ongoing innovations in drug delivery systems are critical for overcoming the challenges associated with peptide therapeutics. Technologies such as nanotechnology, microparticles, liposomal formulations, and implantable devices enhance peptide stability, prolong circulation time, and improve tissue-specific targeting, thereby optimizing therapeutic efficacy and patient compliance.
Competitive Landscape
Leading Companies
Several pharmaceutical and biotechnology companies are actively engaged in the development and commercialization of peptide therapeutics. Notable players include:
- AstraZeneca: Known for its innovative peptide-based treatments in oncology and metabolic disorders.
- Novo Nordisk: A leader in peptide therapeutics for diabetes management, particularly with its GLP-1 analogs.
- Eli Lilly and Company: Focuses on peptide drugs for endocrine and cardiovascular diseases.
- Amgen: Invests in peptide research for cancer and bone health therapies.
Strategic Collaborations and Partnerships
Collaborations between pharmaceutical companies, research institutions, and biotechnology firms are common in the peptide therapeutics market. These partnerships are crucial for pooling resources, sharing expertise, and accelerating the development of novel peptide drugs.
Peptide Therapeutics Market Leading Companies
- Eli Lilly & Company
- Amgen Inc.
- Pfizer, Inc.
- Ever Neuro Pharma GmbH
- Bausch Health
- Abbvie
Segments Covered in the Report:
By Application
- Gastrointestinal Disorder
- Metabolic Disorder
- Neurological Disorder
- Cancer
- Others
By Type
- Generic
- Innovative
By Route of Administration
- Oral
- Parenteral
- Pulmonary
- Mucosal
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Drug Stores
By Synthesis Technology
- Solid Phase Peptide Synthesis (SPPS)
- Liquid Phase Peptide Synthesis (LPPS)
- Hybrid Technology
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/2548
