Lung Stent Market Overview
The Lung Stent Market is a crucial segment within respiratory care, primarily focused on providing solutions for maintaining airway patency in patients with various airway conditions. These stents are typically used to treat conditions such as tracheobronchial strictures, benign or malignant airway obstructions, and post-surgical airway complications.
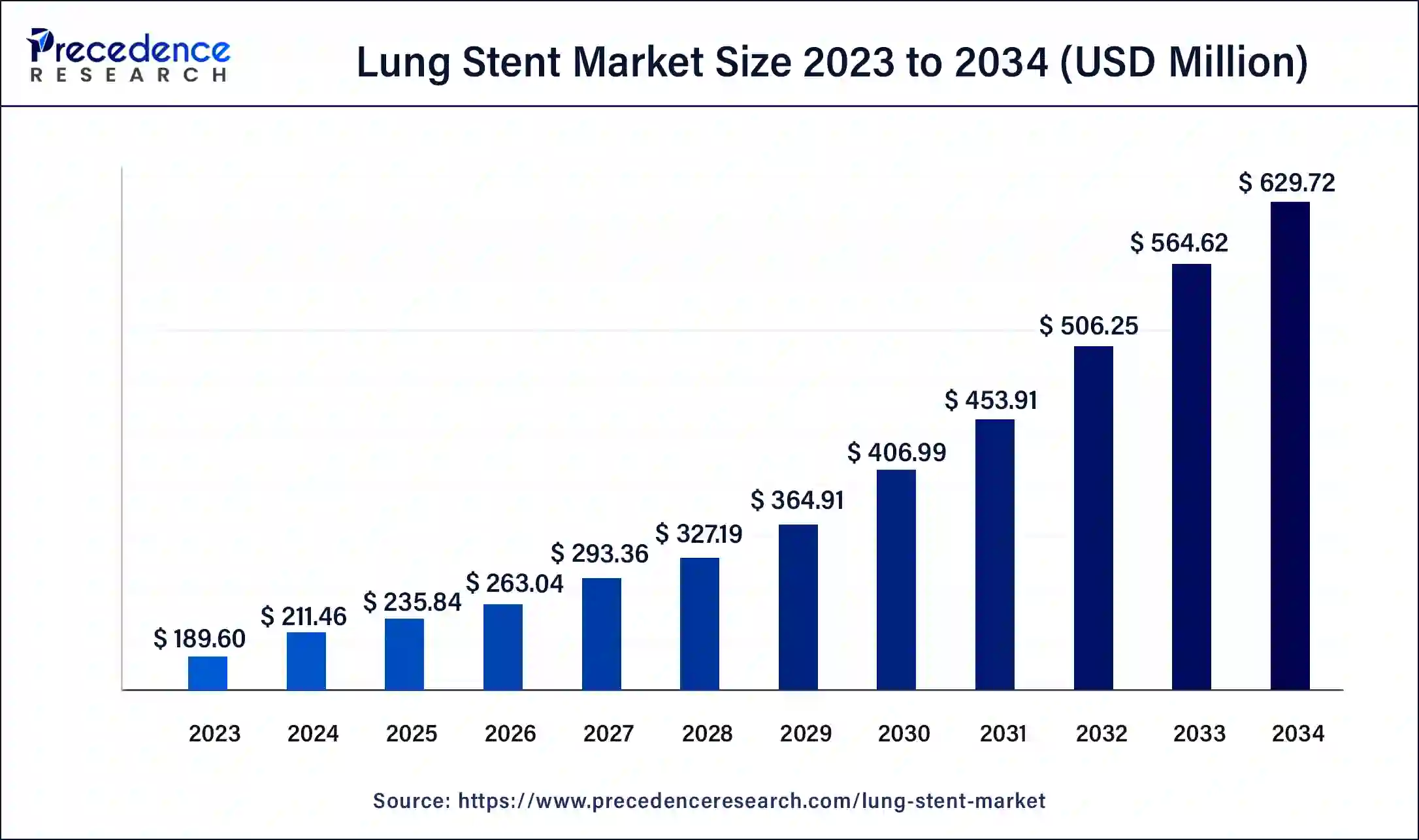
The global lung stent market size exceeded USD 189.60 million in 2023, and it is projected to grow from USD 211.46 million in 2024 to over USD 629.72 million by 2034. From 2024 to 2034, it is anticipated to expand at a compound annual growth rate of 11.53%.
Lung Stent Market Consumer Preferences and Behaviour
1. Price Preference
- Average Cost: The average price of lung stents ranges from $500 to $2,000 per unit. For instance:
- Bronchial Stents: Typically range from $1,000 to $1,500.
- Tracheobronchial Stents: Can cost between $1,500 and $2,000.
- Price Sensitivity: In regions like the US and Europe, healthcare systems often absorb higher costs through insurance or public health funds. In contrast, in countries like India or Brazil, where healthcare budgets are tighter, cost-effective options can be preferred.
2. Buying Patterns
- Hospital Procurement:
- Bulk Purchases: Hospitals often buy lung stents in bulk. For example, a large hospital network might purchase around 1,000-2,000 stents annually, with prices negotiated based on volume.
- Brand Loyalty: According to market reports, around 70% of hospitals in developed markets prefer established brands due to their reliability and clinical evidence.
- Regional Variations: In emerging markets, up to 40% of stents purchased might be lower-cost or generic versions due to budget constraints.
Get a Sample: https://www.precedenceresearch.com/sample/4919
3. Cultural Preferences
- Regulatory Influence:
- US Market: Devices must be approved by the FDA, influencing the choice of stents. As of 2023, about 60% of stents used are from FDA-approved brands with strong clinical data.
- European Market: CE-marked stents are preferred, with approximately 55-65% of stents coming from European-approved manufacturers.
- Awareness and Education:
- Developed Countries: About 75% of healthcare providers are likely to choose high-tech, higher-priced stents based on clinical outcomes and patient safety.
- Emerging Markets: Cost-effectiveness is crucial, with up to 50% of consumers opting for more affordable stents if available.
Read Report: Commercial Robots Market Size to Grow at 20.01% From 2024 to 2034
Brand Preference and Market Penetration
- Leading Brands:
- Boston Scientific: Holds approximately 25% of the global market share.
- Cook Medical: Captures around 20% of the market.
- Medtronic: Accounts for about 15% of the market.
- Emerging Brands: Companies like Becton Dickinson and Conmed are gaining market share with innovative and cost-effective solutions.
Lung Stent Market AI Integration and its Impact
1. Personalized Treatment Planning
- Custom Stent Design: AI is facilitating the development of personalized lung stents tailored to the anatomical structure of a patient’s airway. By leveraging 3D imaging and AI-driven modeling, manufacturers can create stents that fit precisely, improving patient comfort and reducing complications.
- Patient-Specific Recommendations: AI systems can analyze patient data, including comorbidities and past medical history, to recommend the most appropriate stent type and procedure, leading to better patient outcomes.
2. Optimization of Manufacturing Processes
- Smart Manufacturing: AI-driven automation is optimizing the manufacturing of lung stents. Predictive maintenance, quality control, and process optimization through AI can reduce production costs and time, leading to more efficient manufacturing lines and consistent product quality.
- Material Innovation: AI is aiding in the discovery of new materials that can enhance the durability, flexibility, and biocompatibility of lung stents. Machine learning models can predict the performance of various materials under different conditions, accelerating the development of advanced stents.
3. Improved Post-Operative Monitoring and Management
- Remote Patient Monitoring: AI-powered wearable devices and sensors can monitor patients post-operatively, detecting signs of complications such as stent migration or airway blockage. Real-time data analysis enables timely interventions, enhancing patient safety and reducing hospital readmissions.
- AI-Driven Decision Support Systems: These systems assist clinicians in managing post-operative care by analyzing patient data to provide evidence-based recommendations, improving the overall management of lung stent patients.
4. Clinical Decision Support and Training
- AI-Enhanced Training Tools: Virtual reality (VR) and AI-based simulations are being used to train clinicians on stent placement techniques. These tools provide realistic scenarios, allowing for repeated practice in a risk-free environment, improving the skill set of healthcare providers.
- Decision Support Systems: AI-driven decision support systems provide real-time guidance during stent procedures, suggesting optimal placement strategies based on the patient’s specific anatomy and the stent’s characteristics.
5. Regulatory and Compliance Benefits
- Regulatory Compliance: AI can streamline the regulatory compliance process by automating documentation and ensuring that stent designs meet regulatory standards. This reduces the time to market for new products and ensures that they adhere to safety requirements.
- Data-Driven Insights for Approval: AI provides robust data analysis capabilities that can generate comprehensive reports on stent performance, which can be used to support regulatory submissions and clinical trials.
6. Market Dynamics and Competitive Landscape
- Faster Innovation Cycles: AI accelerates the research and development process, allowing companies to innovate faster and bring new products to market more quickly. This is reshaping the competitive landscape, with companies leveraging AI gaining a significant advantage.
- Cost Reduction: AI integration leads to cost savings in manufacturing, R&D, and clinical operations, making lung stents more affordable and accessible, potentially increasing market penetration.
Regional Insights:
-
- North America: The largest market, accounting for about 40% of the global market share in 2023. The high prevalence of lung cancer, chronic obstructive pulmonary disease (COPD), and the presence of advanced healthcare infrastructure are key factors driving growth in this region.
- Europe: The second-largest market, with a significant share driven by high healthcare expenditure, an aging population, and increasing adoption of advanced medical technologies.
- Asia-Pacific: Expected to be the fastest-growing region due to the rising prevalence of respiratory disorders, increasing healthcare spending, and growing awareness of minimally invasive treatments.
Key Market Drivers
- Rising Prevalence of Respiratory Diseases: The increasing incidence of lung cancer, COPD, and other respiratory conditions is a primary driver. According to the World Health Organization (WHO), COPD is the third leading cause of death globally, contributing to over 3 million deaths annually.
- Technological Advancements: Innovations in stent materials (such as silicone and hybrid stents), design improvements (e.g., drug-eluting and self-expanding stents), and AI integration in stent placement and management are enhancing patient outcomes and expanding market applications.
- Growing Preference for Minimally Invasive Procedures: There is a shift towards minimally invasive techniques for treating airway obstructions, driven by shorter recovery times, reduced hospital stays, and fewer complications compared to traditional surgical methods.
- Aging Population: The global aging population is contributing to an increase in respiratory diseases, leading to a higher demand for lung stenting procedures.
- Expanding Indications for Use: Lung stents are increasingly being used not only for malignant obstructions but also for benign airway conditions, tracheomalacia, and stenosis, broadening the market scope.
Market Challenges
- High Costs and Limited Reimbursement: The cost of stents and the associated procedures can be high, and limited reimbursement in some regions can restrict market growth.
- Complications and Safety Concerns: Complications such as stent migration, granulation tissue formation, and infection remain challenges, affecting the overall adoption rates among physicians.
- Stringent Regulatory Requirements: Lung stents must meet rigorous regulatory standards, and obtaining approvals can be time-consuming and costly, which can slow down the introduction of new products.
Key Players in the Market
Some of the leading companies in the Lung Stent Market include:
- Boston Scientific Corporation: A major player offering a range of stents, including the popular Alair™ Bronchial Thermoplasty System.
- Cook Medical: Known for its Evolution® series of bronchial and tracheal stents, widely used in clinical practice.
- Taewoong Medical Co., Ltd.: Provides innovative stent solutions, including silicone and metallic stents tailored for specific patient needs.
- Micro-Tech (Nanjing) Co., Ltd.: A key manufacturer of advanced airway stents, particularly focusing on self-expanding metal stents.
- Novatech SA: Specializes in silicone stents, which are highly preferred due to their biocompatibility and ease of removal.
Emerging Trends
- Biodegradable Stents: The development of biodegradable stents is a significant trend, aimed at reducing complications associated with long-term stent implantation.
- Drug-Eluting Stents: These stents release anti-inflammatory or anti-proliferative drugs to prevent complications like granulation tissue formation and stent re-narrowing.
Segments Covered in the Report
By Products
- Tracheal Stents
- Bronchial Stents
- Laryngeal Stents
By Material
- Metal
- Stainless
- Nitinol
- Silicone
- Hybrid
By End-use
- Hospitals
- Ambulatory Surgical Centers
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/4919
