Market Size and Growth Projections
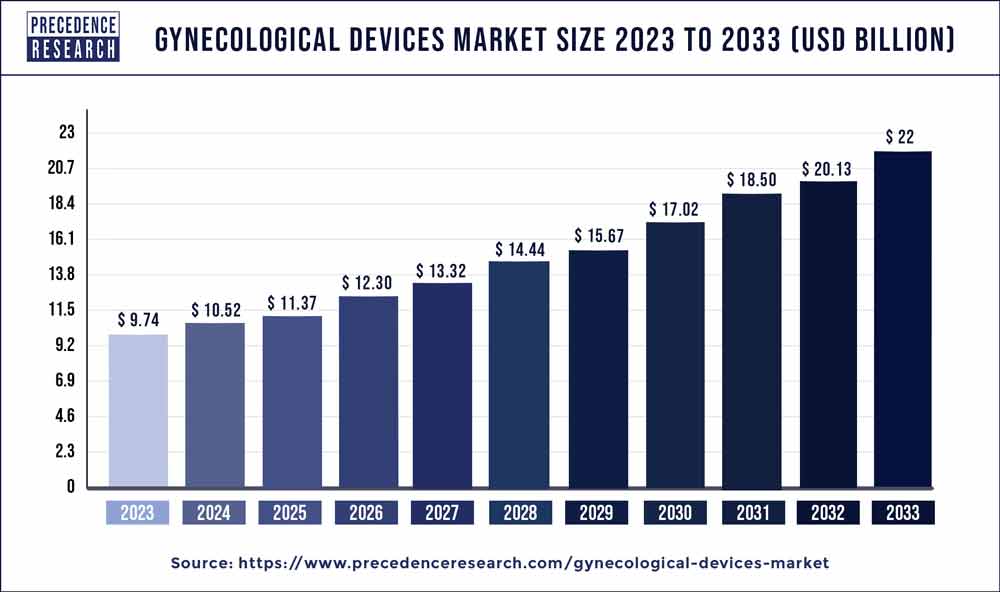
The global gynecological devices market was valued at approximately USD 9.74 billion in 2023 and is expected to reach around USD 22 billion by 2033, growing at a compound annual growth rate (CAGR) of 8.50% during the forecast period. This growth is fueled by several factors, including technological advancements, increasing incidences of gynecological disorders, rising healthcare expenditure, and an aging population.
Introduction
The gynecological devices market encompasses a wide range of products used in the diagnosis, treatment, and management of gynecological conditions. These devices are essential for addressing women’s health issues, including reproductive health, pregnancy, childbirth, and other gynecological disorders. The market has witnessed significant growth in recent years, driven by advancements in technology, an increasing prevalence of gynecological diseases, rising awareness about women’s health, and the expansion of healthcare infrastructure globally.
Get a Sample: https://www.precedenceresearch.com/sample/2523
Key Market Drivers
- Technological Advancements: Innovations in gynecological devices, such as minimally invasive surgical techniques, advanced imaging systems, and robotic-assisted surgeries, have significantly improved the diagnosis and treatment of gynecological conditions. These advancements enhance patient outcomes and reduce recovery times.
- Prevalence of Gynecological Disorders: The increasing prevalence of gynecological disorders such as uterine fibroids, endometriosis, and cervical cancer drives the demand for effective diagnostic and therapeutic devices.
- Rising Awareness and Healthcare Expenditure: Growing awareness about women’s health issues and increasing healthcare expenditure by governments and private entities support the adoption of advanced gynecological devices.
- Aging Population: The global aging population contributes to the rising incidence of gynecological disorders, necessitating the use of various gynecological devices for diagnosis and treatment.
- Expansion of Healthcare Infrastructure: The expansion of healthcare infrastructure, particularly in developing regions, enhances access to gynecological care and boosts the demand for related devices.
Read Report: Patient Monitoring Devices Market Size to Reach USD 89.75 Bn By 2032
Market Segmentation
By Product Type
- Surgical Devices: This segment includes devices used in gynecological surgeries, such as hysterectomy devices, endometrial ablation devices, and gynecological laparoscopy devices. The shift towards minimally invasive surgeries is driving the growth of this segment.
- Diagnostic Imaging Systems: Devices such as ultrasound systems, mammography devices, and MRI systems fall into this category. These imaging systems are crucial for the early detection and diagnosis of gynecological conditions.
- Gynecological Chairs: These are specialized chairs used in gynecological examinations and procedures. They are designed for patient comfort and ease of access for healthcare providers.
- Hand Instruments: This segment includes various instruments used in gynecological procedures, such as forceps, curettes, and specula. They are essential for routine examinations and minor surgical procedures.
- Fluid Management Systems: These systems are used in hysteroscopic procedures to maintain a clear view of the uterine cavity and manage fluid balance. They are vital for ensuring the safety and effectiveness of such procedures.
By End-User
- Hospitals and Clinics: Hospitals and clinics are the primary end-users of gynecological devices, owing to the high volume of gynecological procedures performed in these settings.
- Ambulatory Surgical Centers: These centers are gaining popularity due to their cost-effectiveness and convenience. They are increasingly adopting advanced gynecological devices for various procedures.
- Diagnostic Centers: Diagnostic centers specializing in women’s health use gynecological imaging systems and other diagnostic devices to provide comprehensive diagnostic services.
- Others: This segment includes specialized gynecology centers, research institutions, and academic hospitals.
By Region
- North America: North America holds a significant share of the gynecological devices market, driven by advanced healthcare infrastructure, high healthcare expenditure, and the presence of key market players. The U.S. is the largest market in this region.
- Europe: Europe is another major market, with countries like Germany, France, and the UK leading in terms of adoption of advanced gynecological technologies. The region’s well-established healthcare system and favorable reimbursement policies contribute to market growth.
- Asia Pacific: The Asia Pacific region is expected to witness the highest growth rate during the forecast period, attributed to the growing population, increasing prevalence of gynecological disorders, and rising healthcare expenditure in countries like China, India, and Japan.
- Latin America: This region is also experiencing growth in the gynecological devices market, driven by improving healthcare infrastructure and increasing government initiatives to enhance women’s health services.
- Middle East and Africa: The market in this region is growing steadily, with a focus on improving healthcare facilities and increasing awareness about advanced gynecological care.
Technological Trends
Minimally Invasive Surgery
Minimally invasive surgical techniques, such as laparoscopy and robotic-assisted surgery, have revolutionized gynecological procedures. These techniques offer several benefits, including reduced pain, shorter hospital stays, quicker recovery times, and smaller scars. Laparoscopic devices and robotic systems enable precise and efficient surgeries, enhancing patient outcomes and satisfaction.
Advanced Imaging Systems
Advancements in imaging technology have significantly improved the diagnosis and treatment of gynecological conditions. High-resolution ultrasound systems, 3D and 4D imaging, and MRI systems provide detailed images, enabling accurate diagnosis and better treatment planning. These imaging systems are essential for early detection of conditions such as ovarian cysts, fibroids, and cancers.
Robotic-Assisted Surgery
Robotic-assisted surgical systems, such as the da Vinci Surgical System, are increasingly being used in gynecological surgeries. These systems offer enhanced precision, flexibility, and control, allowing surgeons to perform complex procedures with minimal invasiveness. Robotic-assisted surgery is particularly beneficial in procedures like hysterectomy, myomectomy, and endometriosis resection.
Telemedicine and Remote Monitoring
The integration of telemedicine and remote monitoring technologies in gynecology is gaining traction. Telemedicine platforms enable remote consultations and follow-ups, improving access to care for women in remote or underserved areas. Remote monitoring devices, such as wearable sensors, allow continuous tracking of vital signs and other health parameters, facilitating timely intervention and better management of gynecological conditions.
Regulatory Landscape
The gynecological devices market is highly regulated to ensure the safety and efficacy of the devices. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other national regulatory authorities play a crucial role in the approval and monitoring of these devices. Compliance with regulatory standards is essential for market players to launch and market their products.
Key Regulatory Agencies
- U.S. Food and Drug Administration (FDA): The FDA regulates medical devices in the United States, ensuring their safety and efficacy before they can be marketed. The FDA’s Center for Devices and Radiological Health (CDRH) oversees the approval process for gynecological devices.
- European Medicines Agency (EMA): The EMA is responsible for the scientific evaluation, supervision, and safety monitoring of medicines and medical devices in the European Union. The agency works in collaboration with national regulatory authorities to ensure compliance with regulatory standards.
- Other National Regulatory Authorities: Various countries have their own regulatory bodies that oversee the approval and monitoring of medical devices, including gynecological devices. These authorities ensure that devices meet safety and efficacy standards before they can be marketed.
Regulatory Challenges
Navigating the complex regulatory landscape can be challenging for market players, especially for new entrants. The stringent regulatory requirements and the need for extensive clinical trials to demonstrate the safety and efficacy of devices can delay product launches. Additionally, ensuring compliance with varying regulatory standards across different regions adds to the complexity.
Competitive Landscape
The gynecological devices market is highly competitive, with several key players dominating the market. Some of the prominent players in the market include:
- Boston Scientific Corporation: A leading player in the gynecological devices market, Boston Scientific offers a wide range of devices for minimally invasive gynecological procedures. The company focuses on innovation and strategic acquisitions to expand its product portfolio.
- Hologic, Inc.: Hologic is known for its innovative diagnostic and surgical solutions in the field of women’s health. The company offers advanced imaging systems, surgical devices, and diagnostic tools for gynecological applications.
- Medtronic plc: Medtronic offers a broad portfolio of gynecological devices, including surgical instruments, imaging systems, and diagnostic tools. The company emphasizes technological advancements and integration with digital health platforms.
- CooperSurgical, Inc.: CooperSurgical specializes in providing medical devices and fertility solutions for women’s health. The company offers a wide range of gynecological devices, including surgical instruments, diagnostic tools, and fertility products.
- KARL STORZ GmbH & Co. KG: KARL STORZ is a leading manufacturer of endoscopic instruments and devices for gynecological procedures. The company is known for its high-quality products and innovative solutions in minimally invasive surgery.
Competitive Strategies
Market players adopt various strategies to maintain their competitive edge and expand their market presence. These strategies include:
- Product Innovation: Continuous innovation and development of advanced gynecological devices are crucial for staying competitive. Companies invest in research and development to introduce new products and improve existing ones.
- Strategic Acquisitions and Partnerships: Mergers, acquisitions, and partnerships are common strategies to expand product portfolios, enter new markets, and enhance technological capabilities. Collaborations with research institutions and healthcare providers also help in developing innovative solutions.
- Geographic Expansion: Expanding presence in emerging markets with high growth potential, such as Asia Pacific and Latin America, is a key strategy for market players. Establishing a strong distribution network and local partnerships helps in penetrating these
